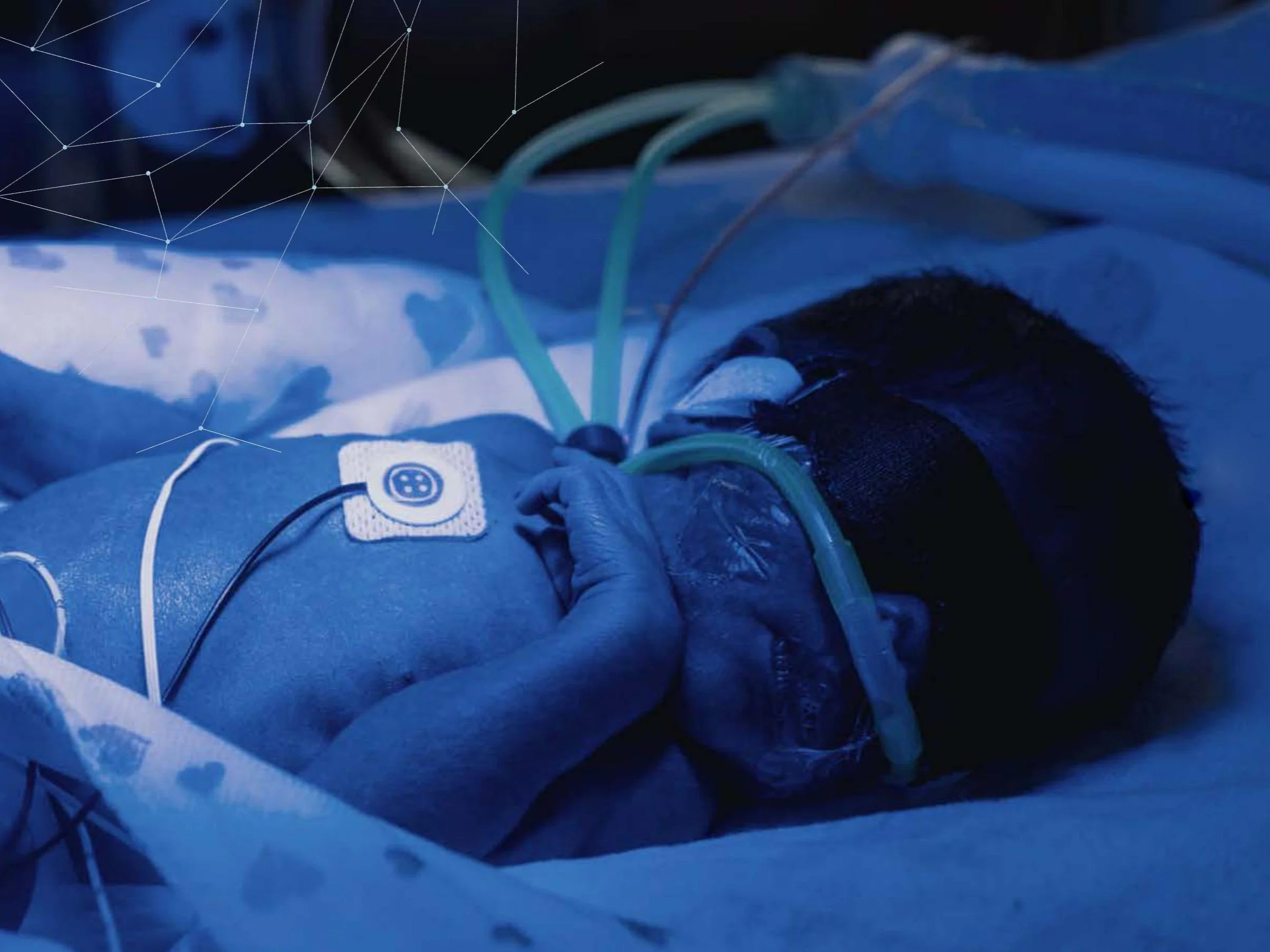
Ventricular hemorrhage is a devastating risk for very premature infants.
Our technology aims to give clinicians an early, actionable warning signal.

HOW WE HELP
During the first days of life, a significant risk of permanent injury exists from ventricular hemorrhage (IVH), caused by rupture of immature blood vessels in the brain due to excessive flow. There is a clear link between excessive fluctuations in blood flow and subsequent development of IVH.
NIRDS 1 is a continuous intracranial blood flow sensor designed to provide real-time monitoring of blood flow, providing clinicians with an early warning signal to potentially mitigate permanent injury.

LIFE-CHANGING TECHNOLOGY
for PREMATURE INFANTS
What is it NIRDS
• Non- invasive real- time cerebral blood flow device
• Technology validated in 1,000+ children
• Exclusive IP license from MassGeneral Hospital
• Currently in clinical evaluation
NIRDS explained
Our probe delivers a small amount of coherent, red light through the skin.
The resulting reflection pattern has a variable intensity, called speckle, that changes from the motion of red blood cells in the field of exposure. We use a computational algorithm to resolve the relative amount of motion that directly correlates to the blood flow.
Now the focus shifts to decreasing disabilities and improving
long-term outcomes.
-
1. Wu et al., 2021 - Validation of Diffuse Correlation Spectroscopy Measures of Critical Closing Pressure against Transcranial Doppler Ultrasound in Stroke Patients
2. Giovanella et al., 2020 - Validation of Diffuse Correlation Spectroscopy against ⁱ⁵O-water PET for Regional Cerebral Blood Flow Measurement in Neonatal Piglets
3. Bangalore-Yoganda et al., 2018 - Concurrent measurement of skeletal muscle blood flow during exercise with diffuse correlation spectroscopy and Doppler ultrasound
4. Proctor et al., 2018 – Validation of diffuse correlation spectroscopy sensitivity to nicotinamide-induced blood flow elevation in the murine hindlimb using the fluorescent microsphere technique
5.Buckley et a., 2012 - Validation of diffuse correlation spectroscopic measurement of cerebral blood flow using phase-encoded velocity mapping magnetic resonance imaging
6. Carp et al., 2010 - Validation of DCS Measurements of Rodent CBF with Simultaneous Arterial Spin Labeling MRI; Toward MRI-Optical Continuous Cerebral Metabolic Monitoring
7. Buckley et al., 2009 - Cerebral Hemodynamics in Preterm Infants During Positional Intervention Measured with Diffuse Correlation Spectroscopy and Transcranial Doppler Ultrasound
8. Durduran et al., 2009 - Transcranial Optical Monitoring of Cerebrovascular Hemodynamics in Acute Stroke Patients
CURRENT STANDARD OF CARE
TRANSCRANIAL DOPPLER ULTRASOUND
● Measures speed not flow
● Not continuous
● Discovers damage after it is irreversible
● Bulky, interferes with other monitoring
• Detects only larger vessels
FUTURE STANDARD OF CARE
NIRDS
● Continuous measurement
● Detects issues early
● Can measure cortical blood flow
● For use in small vessels
ABOUT US
149 was formed to address this clinically vital indication of reducing morbidity and mortality in premature infants.
-
Dr. Franceschini is an Associate Professor at Harvard Medical School with specific training and expertise in the development of non-invasive optical techniques and applications in neuroscience, neurology, and brain health. She received her PhD in Physics from the University of Florence, Italy. With over 100 peer-reviewed journal articles, an H-Index 57, and over 15 patents, she has been a member of the Optics Division of the Martinos Center at Massachusetts General Hospital since 2000. She is widely recognized as a leader in the field of diffuse optical imaging in both neuroscience and clinical neuro-monitoring applications. As a pioneer in the field of near infrared spectroscopy (NIRS), she has made substantial contributions to the development of NIRS instruments and to the modeling and testing of diffusion theory to describe light propagation in turbid media. She has successfully applied the technology to a large range of functional neuroimaging and clinical neuro-monitoring applications. Included in her many distinctions are President of the Society for Functional Near-Infrared Spectroscopy, Fellow of AIMBE and OSA, Distinguished Investigator, Academy of Radiology Research
-
David is is Director of the Neurophotonics Center at Boston University. He received his BS in Physics at Rensselaer Polytechnic Institute and PhD in Physics at the University of Pennsylvania. During his academic career, he has supervised more than 50 students and post-doctoral fellows, and he has published over 300 papers that have received over 40,000 citations and an h-index of 109. He is the founding President of the Society for Functional Near Infrared Spectroscopy and founding Editor-in-Chief of the journal Neurophotonics published by SPIE. Dr. Boas was awarded the Britton Chance Award in Biomedical Optics in 2016 for his development of several novel, high-impact biomedical optical technologies in the neurosciences, as well as following through with impactful application studies, and fostering the widespread adoption of these technologies. He was elected a Fellow of AIMBE, SPIE, and OSA in 2017
-
Jon’s career spans more than forty years of R&D, operations, executive leadership and board experience, in the medical device industry. He has an extensive record of successfully building and leading teams to develop, scale and commercialize a broad array of pioneering products and technologies. More...
Recently, Jon was a Team Lead in NIH’s RADx Tech, a $1B+ program that accelerated dozens of new products to diagnose SARS COV-2.
Prior to that, as President & CEO at Neograft Technologies, Jon and his team developed a technology that created a conformal external support around saphenous vein bypass grafts, raising $40 million, completing three clinical studies and achieving 20+ US patents. He was president and CEO of LumeRx, before that; focused on an endoscopic phototherapy system to treat ulcer-causing H pylori bacteria.
Jon led R&D and operations at several companies before that including Biosphere Medical (sold to Merit) focused on vascular embolization; Urologix (ULGX), focused on microwave ablation for treating BPH, Schneider (BSX), focused on vascular interventional therapeutics. Jon spent 7 years at Boston Scientific’s precursor, Medi-Tech , developing dozens of early percutaneous interventional catheters.
Jon has a B.S. in Physics from the University of Vermont.
-
Bernhard Zimmerman, PhD is a co-founder of 149 Medical and a post-doctoral research fellow at the Neurophotonics Center at Boston University.
Over the past ten years his research activity has been focused on the development of non-invasive optical techniques in brain health and breast cancer detection and therapy monitoring.
Together with Drs. Franceschini and Boas at the Martinos Center for Biomedical Imaging, he built several near-infrared spectroscopy (NIRS) and diffuse correlation spectroscopy (DCS) instruments, covering the complete development cycle from design, through engineering and manufacturing, to programming and performance testing.
Previously he has also gained experimental experience, when he was part of a project to measure functional brain activation using functional near-infrared spectroscopy (fNIRS) in premature newborns to establish a normal baseline. His tasks included study design, data collection in the neonatal intensive care unit, and finally data processing and evaluation.
Bernhard holds a MSc in Electrical Engineering and Information Technology from ETH Zurich, as well as a Ph.D. in Electrical Engineering and Computer Science from the Massachusetts Institute of Technology.
-
Peter Ohanian has more than 40 years of industry experience in medical device quality assurance and regulatory affairs. He has an extensive background developing product clearance strategies and establishing compliance programs for medical devices.
Peter has held various strategic roles in industry, including Vice President of Quality and Regulatory Affairs for Philips Healthcare. Peter has also held similar roles with ZOLL Medical Corporation and Visualization Technology Corporation. He has proven expertise in getting high quality products to market, implementing effective and efficient quality systems, and solving compliance problems. Peter has also served on the Board of Directors for MassMedic.
Peter earned a Bachelor of Science in Biology from Boston College and a Master of Science in Engineering Management from Northeastern University. He is also Regulatory Affairs Certified by the Regulatory Affairs Professional Society.
-
Chris is a certified public accountant and has has more than 25 years of professional experience advising clients on financial, accounting, compliance and operational matters, ranging from strategic planning and risk management to accounting systems and internal control structure.
In addition, he has provided services to enhance, supplement and execute a number of key business activities at his clients, including: treasury operations, financial administration, staff development, board governance, capital management, federal compliance, IT performance, internal & external reporting, vendor management and initial public offering readiness.
He has honed his skills by serving a wide variety of clients in the education, research, engineering, healthcare, manufacturing, financial and technology industries.
A graduate of the Carroll School at Management at Boston College, Chris became a Partner at a Big 5 public accounting firm, and thereafter established a private clients practice providing business advisory, management consulting and outsourced executive services. In providing these services, Chris has functioned as an operating head at some of his clients, including the positions of Chief Financial Officer, Controller, Treasurer and Directors of Accounting, Compliance and Internal Audit.
Contact us
17 Briden Street, Worcester, MA 01605